Authors: Aaron GU丨Pengfei YOU丨Duzhiyun ZHENG丨Matt ZHANG丨Franky YU丨Leyi WANG[1]
China's National Medical Products Administration (NMPA) released the 2023 Drug Review Annual Report on its official website on February 4, 2024, closely followed by the 2023 Medical Device Registration Annual Report on February 5. According to the public information, more than 60 innovative drugs have been approved by NMPA from 2022 to 2023, and a total of 61 innovative medical devices have been approved in 2023. China has been making steady progress in research and development of innovative drugs and medical devices.
By observing each year's drug review reports and medical device registration reports, we can visualize the outstanding achievements of China's regulatory authorities on medical products, which may help us to take a closer look at the development dynamics of China's pharmaceutical industry.
In previous articles, we have made a comprehensive analysis of the drug review annual reports from 2015 to 2021 (See Overview of China Drug Review Annual Report (2015–2021)). In this article, we will analyze the following 2022–2023 drug review reports and the 2023 medical device registration report and select some of the highlights of these reports to observe the major development and achievements of China's drug and medical device regulation activities in the past two years.
The number of registration applications has increased from year to year
I. Drugs
From 2022 to 2023, the number of drug registration applications filed in China has increased year by year. In 2022, the Center for Drug Evaluation (CDE) accepted 12,368 applications. In 2023, the total number of applications has reached 18,503, increasing by 35.84% compared to that in 2022.
Among all drug registration applications, chemical drugs have always been the category at the largest proportion, reaching 74.66% in 2023. However, the number of registration applications for Class 5.1 chemical drugs (i.e., innovator drugs and modified drugs that have been approved to market overseas and are under application to be marketed in China) in 2023 has decreased compared to that of previous years.
The number of registration applications for biological products experienced a slight decrease in 2022 but rebounded in 2023, reflecting the overall trend of continuous development and innovation in China's biological products industry. With the rapid development of modern biotechnology, biological products are consistently achieving innovative breakthroughs. In recent years, the vigorous development of ADC drugs, RDC drugs, mRNA drugs, as well as cellular and gene therapy (CGT) products such as CAR-T/NK/TIL, among others, has brought forth optimism within the pharmaceutical industry.
II. Medical devices
In 2023, the total number of registration applications for medical devices has increased by 25.4% compared to that in 2022. Among them, in terms of medical device classification, NMPA accepted 7,106 domestic Class III medical device registration applications and 6,154 imported medical device registration applications (including 3,036 Class II medical devices and 3,118 Class III medical devices). In terms of product varieties, there were 9,968 medical device registration applications and 3,292 in vitro diagnostic reagents (IVD reagents) registration applications. Registration applications for IVD reagents constituted 24.80% of all applications in 2023, marking a significant growth in both quantity and percentage compared to those in 2022.
The completion of application review is rising steadily
I. Drugs
According to the 2022 Drug Review Annual Report, there was a decline in the number of completed application reviews in 2022 compared to those of previous years. This decrease was mainly attributed to the widespread disruption of drug research and development activities resulting from the COVID-19 pandemic, which also led to the impact on the progress of the review and approval of the drugs.
Fortunately, the year 2023 witnessed a rebound in the number of completed application reviews, with a total of 15,713 completed applications, reaching the highest among recent years.
II. Medical devices
In 2023, NMPA approved a total of 12,213 applications for medical device registrations, marking a 2.3% rise from 2022. Among the approved registrations, in terms of medical device classification, 6,151 were domestic Class III medical devices and 6,062 were imported medical devices (comprising 2,947 Class II medical devices and 3,115 Class III medical devices). The approval rate for imported medical devices witnessed a 3% decrease compared to that of 2022. In terms of product varieties, 9,130 medical device registration applications were approved, and 3,083 IVD reagent registration applications were approved. Approvals of IVD reagents have increased in both quantity and percentage compared to those in 2022.
Various pathways accelerate the marketing of innovative and high-quality products
I. Drugs
In 2022–2023, CDE continued to shorten the time for drug development and review by implementing various accelerated procedures for drug registration, facilitating the marketing of more innovative and high-quality products while broadening treatment options available to patients. It is worth mentioning that the accelerated procedures have played an obvious role in the marketing of innovative drugs: among the 40 innovative drugs approved in 2023, 9 of them were approved through the priority review and approval procedure, 13 obtained conditional marketing approval, and 8 were incorporated into the breakthrough therapy drug procedure during the clinical research stage. The drugs registered and approved through the above pathways are mainly antitumor drugs.
In China, various accelerated pathways for drug marketing registration include:
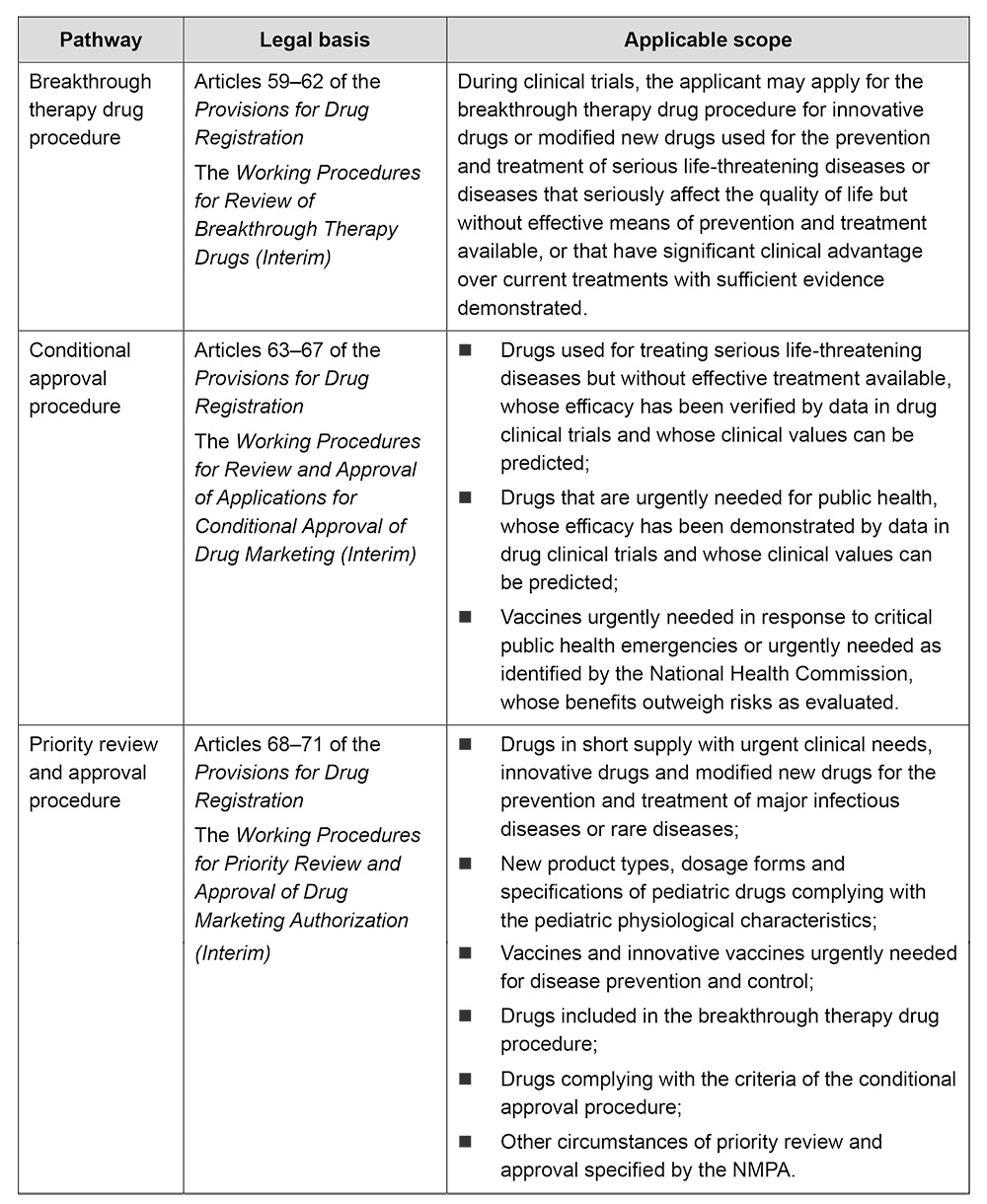
II. Medical devices
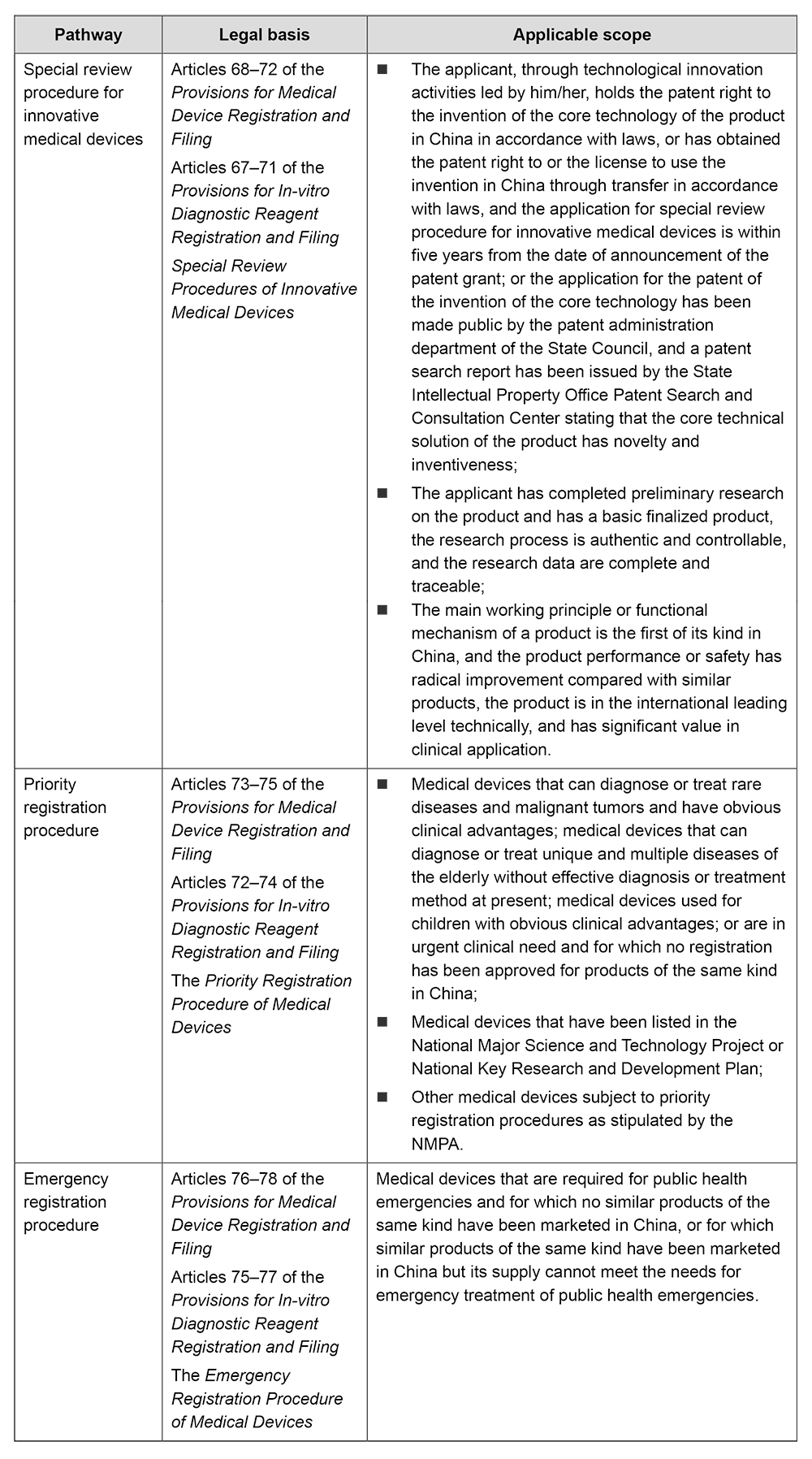
In 2023, NMPA received 466 applications for the special review procedure for innovative medical devices, marking an increase of 35.9% compared to the number in 2022. Among them, 69 applications were accepted by the special procedures.
China has set up a number of accelerated pathways for various medical device products, such as the special review procedure for innovative medical devices, the priority registration procedure and the emergency registration procedure. These pathways have played an important role in accelerating the marketing of innovative and high-quality products to meet the multifaceted needs of patients.
The development and marketing of innovative products are supported and encouraged
I. Drugs
Since the reform of the review and approval system for drugs and medical devices, China has been attaching great importance to encouraging innovative development in the field of pharmaceuticals and has formulated numerous policies to support and encourage more innovative drugs to be developed and registered. Due to the support from regulatory policies, the research and development of innovative drugs in China has been very active in recent years. From 2022 to 2023, the number of applications and approvals for innovative drugs including traditional Chinese medicines, chemical drugs, and biological products has increased by varying degrees. For example, the number of NDA applications and approvals for innovative chemical drugs has increased by 172.41% and 123.53% respectively, while the number of NDA applications and approvals for innovative therapeutic biological products has increased by 136.84% and 111.11% respectively.
II. Medical devices
In 2023, China's innovative medical devices have made significant progress in terms of both quantity and quality. As mentioned above, NMPA received 466 applications for the special review procedure for innovative medical devices in 2023, and 69 applications were accepted to enter the special review procedure. It has also been mentioned in the annual report that a total of 6l innovative medical devices were approved by the NMPA in 2023, increasing by nearly 11% from 2022. High-end medical devices such as active surgical devices, passive implantable devices, medical software, medical imaging devices, and radiation therapy devices are among the top five category in number of approved innovative medical devices.
While the quantity is increasing, the quality of China's innovative medical devices is also improving. A number of innovative medical devices have reached the international leading level, effectively meeting the public's demand for high-end medical devices.
NMPA is striving to meet the patients' multifaceted medication needs
In each year's drug review annual reports, NMPA has consistently paid great attention to special products such as drugs for pediatric use and orphan drugs, of which China has made significant progress in the review and approval procedures in recent years.
I. Drugs in short supply
In 2020, China issued the National List of Drugs in Short Supply for the purpose of solving the problem of shortages on the supply side of drugs and guarantee a more stable supply of drugs. It is provided in the 2023 Drug Review Annual Report that CDE recommended the approval of a total of 82 drugs on the National List of Drugs in Short Supply in 2023, an increase of 310% compared with that of 2022.
II. Drugs for pediatric use
From 2019 to 2023, the number of approvals for drugs for pediatric use has been increasing year by year. In 2023, 92 drug varieties were approved, including 72 for marketing authorization and 20 with extended indications for children.
Since 2016, China has formulated four batches of lists of drugs for children to promote the development and registration of the varieties, dosage forms and specifications suitable for children. From 2022 to 2023, CDE has together recommended the approval of 22 generic drugs on the above-mentioned list. In the past five years, China has cumulatively recommended the approval of 45 generic drugs on the list, fulfilling the needs of pediatric patients in various aspects.
III. Orphan drugs
In 2023, a total of 45 drug varieties for rare diseases were approved for marketing in China, of which 15 varieties (33.3%) were accelerated through the priority review and approval procedure and one product was approved for marketing through the conditional approval procedure. CDE’s priority review resources have been tilted towards the registration applications of orphan drugs year by year.
IV. CGT products
In recent years, China's biomedical technology has experienced rapid growth with new breakthroughs. The new generation of therapies represented by cell therapy and gene therapy is maturing. In 2023, China approved three CAR-T cell therapy products, including the conditional market approval of the Equecabtagene Autoleucel Injection and the Inaticabtagene Autoleucel Injection, and the conditional approval for the addition of new indications to Axicabtagene Ciloleucel Injection.
CDE also issued a number of guidelines and documents related to the development of the CGT products in 2023 in order to better guide the research and registration of the CGT products.
It is worth noting that China's National Health Commission has also been actively exploring the regulatory models for CAR-T/NK and other somatic cell therapies in recent years. On August 18, 2023, the China Medicinal Biotech Association (CMBA), commissioned by the National Health Commission, issued the Guidelines for the Somatic Cell Clinical Research (for Trial Implementation), which provides the industry with compliance guidelines to continuously explore and develop cell therapy technology.
Policies and applications of real-world research have further developed
In recent years, in order to accelerate the marketing of innovative drugs and medical devices, China has been exploring the policy of applying real-world data in the market registration of drugs and medical devices. A number of related guidelines have been issued and implemented.
In 2019, China launched a pilot project on the application of clinical real-world data in the Hainan Boao Lecheng International Medical Tourism Pilot Zone, demonstrating China's attempts in the field of drug and medical device regulation. The pilot project has achieved remarkable results. To date, four drugs and nine medical devices have been approved through the pilot project, benefiting more and more patients.
Regulatory guideline system becomes more mature
I. Drugs
In the process of drug development and registration application, the applicants often need to communicate and discuss with CDE on the specific requirements of the development of the products. Conducting communication meetings with the applicants is an important measure for CDE to guide and serve the applicants.
In 2023, CDE received a total of 5,912 requests for communication meetings, increasing by 20.06% compared to that of 2022, and CDE cumulatively provided communication services to l,607 enterprises. Communication meetings are becoming more and more common as a bridge of communication between the applicants and the regulatory authorities, and the services provided by the regulatory authorities are becoming increasingly adequate.
In addition, to better guide the development and registration activities of the applicants, CDE has formulated and issued respectively 61 and 60 new technical guidelines in 2022 and 2023. Up to now, the cumulative number of drug-related technical guidelines issued by CDE has reached 482. The guidelines issued in recent years cover a wide range of contents, including the evaluation system of CGT technology, the policies regarding real-world research, and other aspects, focusing on international cutting-edge technology areas to promote the integration of China's guideline system with the international advanced technology standards.
II. Medical devices
In 2023, CMDE issued a total of 67 guidelines and 6 review points, bringing the number of active guidelines to 613. The issuance of a large number of guidelines helps to continuously promote the standardization of the research and development activities and the review activities.
Summary
Looking back at the drug and medical device review and approval activities in China in 2022–2023, it can be found that over the years China's reform of the review and approval system has achieved remarkable results. From the perspective of product development, the quantity and quality of products for market registration have been substantially improved in recent years. From the perspective of product regulation, the review and approval activities by the regulatory authorities have become more standardized, organized and efficient, better catering to the needs of applicants. A mature regulatory system will escort the development and marketing of more innovative, high-quality products, and the common progress of technology and regulatory system will combine to promote the vigorous development of China's pharmaceutical industry, so that more patients can enjoy more products with better quality.
Important Announcement |
|
This Legal Commentary has been prepared for clients and professional associates of Han Kun Law Offices. Whilst every effort has been made to ensure accuracy, no responsibility can be accepted for errors and omissions, however caused. The information contained in this publication should not be relied on as legal advice and should not be regarded as a substitute for detailed advice in individual cases. If you have any questions regarding this publication, please contact: |
|
Aaron GU Tel: +86 21 6080 0505 Email: aaron.gu@hankunlaw.com |
[1] Shuwen Sun have contributions to this article.