Authors: Aaron GU丨Pengfei YOU丨Duzhiyun ZHENG丨Fengqi YU[1]
On June 1, 2023, China's Ministry of Science and Technology ("MOST") officially released the Implementation Rules for the Regulation of Human Genetic Resources Administration ("Implementation Rules"), which will come into effect on July 1, 2023. Since the release of the Rules for Implementation of the Regulations on Administration of Human Genetic Resources (Draft for Comments) in March 2022 by MOST ("Draft Rules"; for reference, please refer to Han Kun • Perspective | Highlights on the Draft HGR Regulations Implementation Rules), the industry has been eagerly awaiting the finalization of the policies outlined in the Implementation Rules. We closely followed the updates and noticed that some changes in the Implementation Rules have already been reflected in the regulatory practice. The long-awaited release of the Implementation Rules marks a new stage in China's regulation of human genetic resources.
The Biosecurity Law enacted in 2020, the Regulations on the Administration of Human Genetic Resources released in 2019 ("HGR Regulations"), and a series of administrative approval/filing service guidelines and regulations issued by MOST in recent years, including Frequently Asked Questions, together form the current overall regulatory framework for human genetic resources in China. However, until now, higher-level regulations such as the HGR Regulations lacked detailed implementation measures to further clarify the regulatory scope. The release of the Implementation Rules further refines China's overall framework for human genetic resources administration and provides more detailed compliance guidelines for the industry to carry out activities utilizing these resources.
This article will analyze and summarize the key provisions of the Implementation Rules such as administration, definition of human genetic resources information, definition of Foreign Parties, ethical review requirements, collection and biobanking requirements, international cooperation requirements, information provision and security review, supervision and administration, and punishment responsibilities.
Administration refinement
I. Delegation of regulatory authority
According to the HGR Regulations, the supervision and administration of human genetic resources in China is jointly carried out by the national competent departments and provincial competent departments in accordance with administrative authority. Meanwhile, the national competent departments are directly responsible for the administrative approval and filing of the collection, biobanking, international cooperation, and provision of human genetic resources to foreign parties. The Implementation Rules further explore the delegation and decentralization of China's HGR regulatory authority, which helps to enrich regulatory resources. Article 3 of the Implementation Rules stipulates that MOST may delegate relevant organizations to carry out formal review and technical review of HGR applications, as well as the administration of filing, prior reporting, supervision, inspection, and administrative penalty of HGR activities. Article 4 clarifies that provincial departments of science and technology, in addition to being responsible for the daily supervision and administration in their respective regions, can also review HGR approvals under the delegation of MOST. It is thus evident that under the Implementation Rules, MOST can delegate its responsibility to review administrative approval to the provincial level and entrust its duties to review filing, supervision, and administration to relevant organizations. For example, by the end of 2022, the Shanghai Science and Technology Commission, with the support of the National HGR Office, established the Shanghai Human Genetic Resources Administration Service Station, based on the Shanghai Biomedical Technology Development Center. The station primarily conducts HGR business consultations, HGR specialist training, and assists in the pre-, in-, and post-event HGR compliance.
II. Competent regulatory authorities
Notably, the Implementation Rules are promulgated by MOST, which is consistent with the long-standing practice of MOST supervising the utilization of human genetic resources in China. However, in March, 2023, the Central Committee of the Communist Party of China and the State Council issued the "Plan for the Reform of the Party and State Council," and decided to re-organize MOST and transfer China Biotechnology Development Center, which is responsible for the daily work of Human Genetic Resources Administration concerning biosecurity and biological resource administration, to be under the jurisdiction of the National Health Commission. This change may lead to the alteration of the competent department for the supervision and administration of human genetic resources in China. However, consistent with the current Biosecurity Law and HGR Regulations, Articles 3 and 4 of the Implementation Rules clearly stipulate that the supervision of HGR is still the responsibility of MOST. We understand that the institutional reform plan is still in the implementation phase, and the upper-level regulatory laws and competent departments for human genetic resources may still change in the future. However, given that the administrative approval and filing requirements for the collection, biobanking, international cooperation, and provision of human genetic resources to foreign parties are stipulated in the Biosecurity Law, the regulation framework of China's human genetic resources will not undergo fundamental changes unless the National People's Congress amends the law.
Definition of human genetic resources information
According to Article 2 of the HGR Regulations, human genetic resources information refers to data and other information materials generated from the use of human genetic resources materials. However, such criterion is relatively broad and brings uncertainties in practice.
In Article 2 of the Implementation Rules, human genetic resources information refers to data and other information such as human genes and genome data generated from the use of human genetic resources materials, excluding clinical data, imaging data, protein data, and metabolic data. This further clarifies the regulatory scope of human genetic resources information in China. The Implementation Rules specify that imaging data such as B-ultrasound and CT are not included in the human genetic resources information, which is consistent with the interpretations in the Q&A released by MOST in March and April 2022. However, it is worth noting that, according to our previous understanding, the regulatory authorities once considered adding "biomarker" data to the definition of human genetic resources information, but this was not included in the finalized Implementation Rules. We understand that biomarkers are not explicitly excluded from the scope of human genetic resources information by authorities, and the regulatory requirements still apply when the biomarker data contains human gene and genome data information. In addition, it remains uncertain whether the data analysis conclusions and other information generated from the research using human genetic resources fall under the scope of human genetic resources information. We understand that, based on the definition of the Implementation Rules, the analysis conclusions and other information materials, in the absence of gene and genome data information, will not be subject to human genetic resources regulatory requirements.
Clear definitions of human genetic resources are the prerequisites for the regulation applications. Only when the regulatory rules properly define human genetic resources can industry practitioners more accurately fulfill their compliance obligations. It can be seen that the current Implementation Rules have returned to the essence of regulating human genetic resources and have reasonably defined the regulatory scope of human genetic information, which is in line with MOST's HGR regulatory principle of "stringent regulation where necessary and reasonable flexibility where appropriate".
Scope of foreign parties
Pursuant to the HGR Regulations, foreign parties are facing more restrictions on utilizing human genetic resources than Chinese parties. For example, foreign parties are prohibited from collection and biobanking of human genetic resources, and it would be necessary to cooperate with Chinese parties and go through approval and filing procedures in order to conduct scientific research utilizing human genetic resources. MOST has always been treating entities with any foreign capital as foreign parties since the Interim Measures for Administration of Human Genetic Resources in 1998. The term "foreign party" was more clearly defined as "foreign organizations and institutions that are established or actually controlled by foreign organizations or individuals" by the current HGR Regulations promulgated in 2019, based on which the Implementation Rules have further improved the standards for recognizing foreign parties.
First, the Implementation Rules clearly emphasize the "50%" ratio for defining foreign parties, and stipulate that circumstances where an overseas organization(s) or an individual(s) establishes or actually controls an institution include: "(1) an overseas organization or an individual holds or indirectly holds 50% or more of the shares, equity, voting rights, property shares or other similar rights and interests of the entity" or "(2) although the shares, equity, voting rights, property shares or other similar rights and interests of the entity directly held by an overseas organization or an individual do not reach 50%, the voting rights or other rights and interests it owns are sufficient to control or have significant influence on the resolutions, decision-making and internal management of the entity." After the Implementation Rules come into effect, an entity will no longer be regarded as a foreign party if overseas organizations or individuals have less than 50% of the equity and have no significant influence on the decision-making or internal management of the entity. This would potentially bring convenience to entities with only limited foreign ownership in utilizing human genetic resources. However, it remains to be seen how the regulatory authorities will determine the term "significant influence" in practice. In addition, the Implementation Rules have unified the criteria for recognizing foreign parties when they are established or actually controlled of overseas organizations and individuals compared with the Draft for Comments to make the provisions more reasonable.
Second, consistent with the Draft for Comments, the Implementation Rules have made it clear that Chinese entities with VIE structures will be recognized as foreign parties. The third paragraph of Article 12 of the Implementation Rules stipulates that "investment, agreements, or other arrangements by an overseas organization(s) and an individual(s) are sufficient to control or have significant influence on the decision-making, internal management and other major matters of an entity". Although pursuant to the HGR Regulations, VIE structures have already been covered as "actual control" in the definition of foreign parties, the Implementation Rules has put an end to the controversy about the nature of entities with VIE structures in practice, and such entities will be required to conduct their business activities related to human genetic resources in compliance with the regulatory requirements for foreign parties.
Third, Article 11 of the Implementation Rules stipulates that entities in Hong Kong and Macau actually controlled by Chinese capital will be regarded as Chinese parties. The definition of "actually controlled by Chinese capital" needs further interpretation from regulatory bodies, and from our point of view, the companies established in Hong Kong and Macau or the subsidiaries in Hong Kong and Macau actually controlled by Chinese companies may fall under the scope of Chinese parties, while as for subsidiaries established in Chinese mainland by Hong Kong and Macau companies, they will not fall under the scope of Article 11 and their status still need to be further examined based on the criteria stipulated in Article 12.
Ethics review requirements
The Implementation Rules have refined the ethics review requirements for the utilization of human genetic resources in China. Article 8 of the Implementation Rules stipulates that the collection, biobanking, utilization, and external provision of human genetic resources in China shall conform to the ethics principles, and the ethics review shall be conducted by the Ethics (Review) Committee, which has been filed by the relevant regulatory authorities. The ethics review shall be carried out in accordance with the law, administrative regulations, and other relevant provisions. Compared with the Draft for Comments, where the specific requirements for ethics review shall refer to the relevant provisions in the Measures for the Ethical Review of Life Science and Medical Research Involving Humans by the National Health Commission, the Implementation Rules generally stipulate that the review should be conducted by the Ethics Committee filed with the relevant regulatory authorities and comply with relevant laws and regulations. From our understanding, the Implementation Rules have left space for the technology ethics review regulations, such as the Technology Ethics Review Method (Pilot Implementation) (Draft for Comments) issued by MOST in in April 2023. Given that the regulations of technology ethics review have not been officially promulgated, it is still uncertain about the application and relationship between the ethics review regulations issued by the Health Commission and the technology ethics regulations. The ethics review requirements in the utilization of human genetic resources in China in the future still need further observation to future legislations (for reference, please refer to Han Kun • Perspective | Technology Ethics Review Method (Trial) (Draft for Comments): a Brief Overview and Han Kun • Perspective | New Ethics Review Regulations: Key Takeaways).
Changes in collection and biobanking requirements
I. Changes in the scope of collection approval
In accordance with the Biosecurity Law of the PRC and the HGR Regulations, the collection of human genetic resources of important genetic families, in specific regions, or the specified categories and quantity shall be approved by relevant regulatory authorities. The Guidelines for the Collection Approval of the Collection of Human Genetic Resources in China (the "Guidelines") further stipulates the specific requirements in scope for the collection approval of human genetic resources. The Implementation Rules have updated the scope requirements for collection approval. Please see below the comparison:
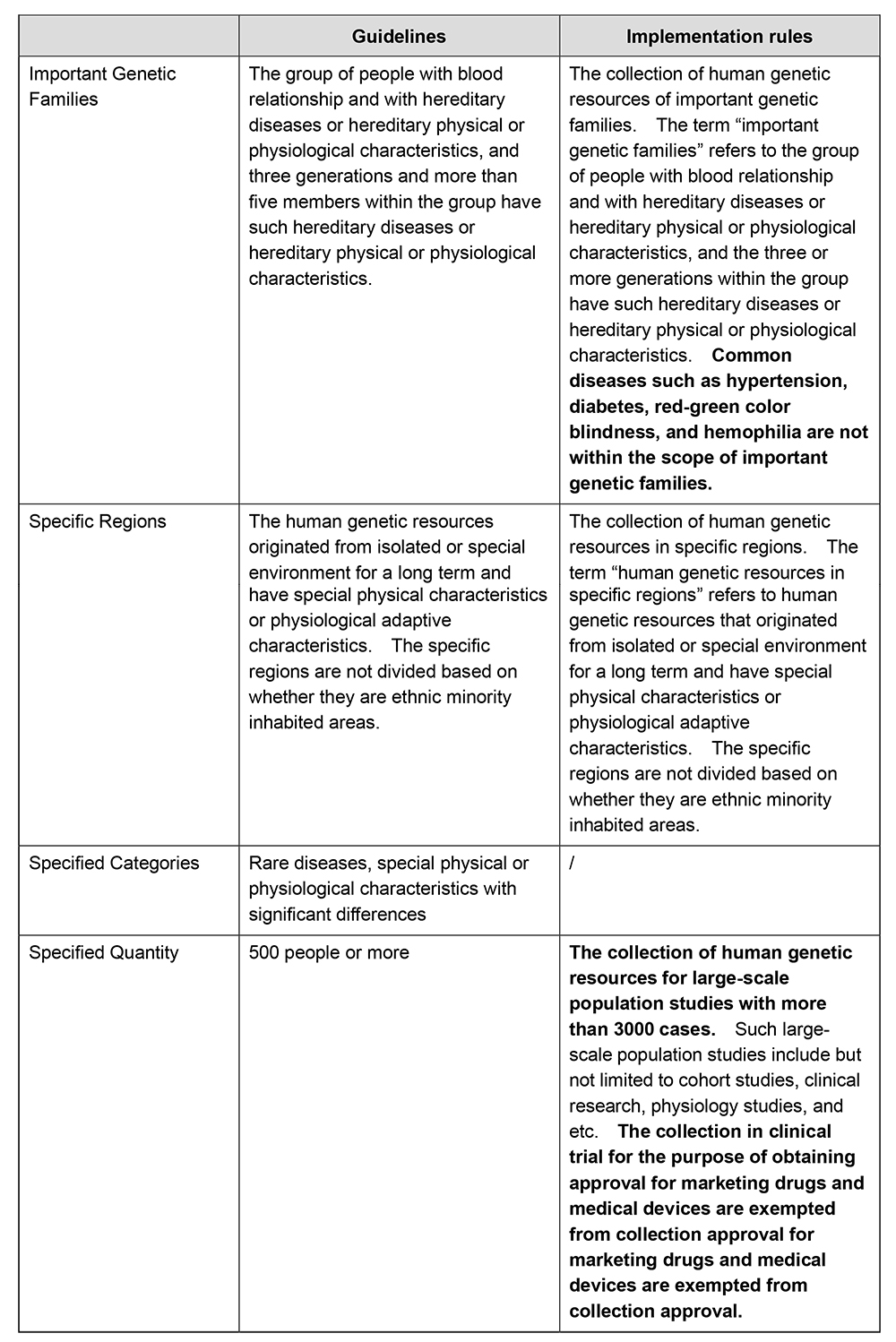
The Implementation Rules have broadened the scope of collection in need of approval, and have clarified that common diseases such as hypertension, diabetes, red-green color blindness, and hemophilia are not within the scope of important genetic families. The Implementation Rules have also eliminated the collection approval requirements for specific categories such as rare diseases, and meanwhile, have raised the minimum requirement for the specified quantity for collection approval. It is stipulated that only collection activities for large-scale population studies with more than 3000 cases need to be declared for collection approval, and the collection in clinical trial for the purpose of obtaining approval for marketing drugs and medical devices are exempted from collection approval, for example, the collection of human genetic resources in international cooperative clinical trials for obtaining marketing approval will no longer need collection approval even though the quantity of collection exceeds 3000. The Implementation Rules will significantly ease the approval and regulatory burden of Chinese parties in collecting human genetic resources and conducting human genetic resource utilization activities and conserve the regulatory resources.
II. Changes in biobanking regulatory requirements
Regarding the regulatory requirements for the biobanking of human genetic resources, the Implementation Rules refined the provisions of the HGR Regulations in terms of the definition of biobanking activities and collection exemption permits. In terms of the definition of biobanking activities, Article 28 of the Implementation Rules stipulates that biobanking activities refer to the behavior of preserving legally obtained human genetic resources under suitable environmental conditions to ensure their quality and safety for future scientific research. It does not include temporary storage activities for teaching purposes or biobanking in accordance with legal requirements or clinical research protocol agreements after lab testing, which is consistent with the provisions of the Chinese Human Genetic Resources Biobanking Approval Administrative Service Guide ("Biobanking Approval Guide"). The Implementation Rules reiterate the distinction between biobanking and temporary storage activities at the regulatory level. Therefore, temporary storage of human genetic resource materials involved in clinical trials or investigator initiated trials (IIT) initiated by sponsors in cooperation with trial sites in practice does not require biobanking approval procedures to be performed. In terms of exemption for HGR collection approval, Article 29 of the Implementation Rules explicitly stipulates that if the requirements for biobanking approval application are met, applicants do not need to additionally apply for collection approval, which simplifies administrative approval regulatory requirements.
In addition, compared with the HGR Regulations and other regulations such as the Collection Approval Guide and the Biobanking Approval Guide, the Implementation Rules clearly stipulate the change procedures for the collection/biobanking of human genetic resource approvals, providing clear compliance guidance.
Section 6: Changes in International Cooperation Regulatory Requirements
Regarding the regulation of international cooperation, the Implementation Rules have detailed provisions on the approval/filing requirements, approval/filing process, and cooperation reporting requirements. Among them, the relaxation of international cooperation filing requirements and the changes in the definition of non-major changes in international cooperation approval are noteworthy. The key points of analysis and introduction are as follows:
1. Relaxation of international cooperation filing requirements for international cooperation
Firstly, the Implementation Rules further expands the scope of international cooperation filing. According to the HGR Regulations, compared with the international cooperation administrative approval process, only the filling process needs to be followed if the following conditions are met:
In order to obtain the marketing license for relevant drugs and medical devices in China;
Conducting international cooperative clinical trials using Chinese human genetic resources in clinical trial sites;
Not involving the export of human genetic resource materials.
However, the HGR Regulations haven't clearly defined the scope of "conducting clinical trials in clinical trial sites". According to the "Guidelines for the Filling of International Cooperative Clinical Trials on Chinese Human Genetic Resources" ("International Cooperation Filing Guidance"), "conducting clinical trials in clinical trial sites" are not limited to the internal processing of human genetic resources in clinical trial sites, but also include the collection of human genetic resources by clinical trial sites and the testing, analysis and disposal of residual samples by parties entrusted by clinical trial sites through written agreements. The Implementation Rules further expands the scope of "conducting clinical trials in clinical trial sites" to include not only internal processing within clinical medical and health institutions but also the collection of human genetic resources by clinical medical and health institutions and the testing, analysis and disposal of residual samples by domestic institutions designated by the clinical trial protocol. In view of this, in the future, the industry can make more flexible cooperation arrangements in international clinical trials, such as the sample testing service entrustment agreement, which does not have to be signed by clinical medical and health institutions and the entrusted parties, but can be signed by the sponsor or even the contract research organization ("CRO") on behalf of the sponsor and the entrusted parties, which is more in line with industry practice
Secondly, the Implementation Rules further clarifies that the human genetic resource information generated from international cooperation can be shared among cooperative partners. Paragraph 3 of Article 28 of the HGR Regulations stipulates that the human genetic resource information generated from the use of China's human genetic resources in international cooperations can be used by both partners. Based on this, Article 36 of the Implementation Rules clearly stipulates that during the implementation of international cooperations that has obtained administrative approval or has completed filings, the Chinese party provides the foreign party with the human genetic resource information generated from the cooperation, if it has been agreed in the international cooperation agreement that the information can be used by both partners, there is no need to submit a separate prior reporting and information backup. This position is consistent with previous regulatory practice and is now reflected in the Implementation Rules.
In addition, the Implementation Rules have added provisions for the change of filing procedure for international cooperation filings. Previously, according to the International Cooperation Filing Guidelines, significant changes in international cooperation required re-filing, while non-significant changes only required uploading an explanation of the change on the platform. The newly introduced Article 53 of the Implementation Rules supplements the provisions for non-significant changes, requiring the uploading of a change report in advance on the platform, and stipulating that the record-keeper should promptly submit change filings in case of significant changes, which is conducive to reducing the compliance burden of applicants.
2. Definition of non-significant changes for international cooperation approval/filing
The HGR Regulations stipulate that changes in major matters such as the cooperating partners, research objectives, research content, and cooperation period of international cooperation activities should be subject to change approval procedures. However, the major changes listed in the HGR Regulations are relatively general and have a broad scope. Therefore, the Implementation Rules provide criteria for non-significant changes, which are useful for practical reference. Non-significant changes mainly include the following situations:
Changes that only involve a total amount not exceeding 10% of the approved amount of HGR without changing the research content or research plan;
Changes in participating parties other than the sponsors, leading sites, CROs, and third-party laboratories;
Changes in the name of the legal entity of the cooperating partner;
Changes in the research content or research plan without changes in the type, quantity, and purpose of human genetic resources or without exceeding the approved scope after the change.
In the case of non-significant changes, the parties only need to submit relevant materials to MOST for explanation and filing, without the need for change approval procedures. Therefore, in situations where the research activities only involve changes within 10% of the approved amount or changes in participating units such as the Electronic Data Collection System (EDC) supplier, the simplified explanation procedure for non-significant changes can be applied.
3. Intellectual property sharing provisions
The Article 17 of the Draft Rules had explicitly stipulated that the cooperation parties could agree on the use rights, transfer rights, and profit-sharing methods of other scientific and technological achievements such as works, data, standards, and process flows generated by international cooperations through agreements. The relevant provisions were deleted in the Implementation Rules. From the perspective of legislative methodology, the Implementation Rules have deleted many overlapping and repetitive provisions in the Draft Rules and the HGR Regulations. Considering the current regulatory positions on patent rights and other intellectual property rights in the HGR Regulations, we understand that unlike patents, research results such as clinical trial data may not necessarily be jointly owned by Chinese and foreign cooperation parties. The Chinese and foreign parties have more autonomy in determining data ownership. Well aware of the value and importance of research data and other information materials for pharmaceutical companies, we understand, on the one hand, such research data is likely to be used to support future drug market authorization submissions to drug regulatory authorities of different jurisdictions; on the other hand, such data may have significant value in pharmaceutical companies’ subsequent licensing and cooperative research and development projects, as an integral part of technology transfer. Furthermore, it may even become the basis for payment of royalties when licensing patents expire, as licensed know-how.
Information provision and security review
A significant change in the regulation of information provision is that the Implementation Rules modified the filing procedure stipulated in the HGR Regulations to a prior reporting procedure. Under the previous filing procedure, in practice, completing the filing procedure for the external provision or open utilization of human genetic resources information usual took several weeks. Since the Implementation Rules adjusts the filing procedure to a prior reporting procedure, it remains to be seen whether the time limits will be shortened.
Furthermore, Section 4 of the Implementation Rules also supplements the regulations for the changing report procedure. Going forward, after a company has completed the backup and reporting procedures for external provision of information, any changes regarding the usage purpose of information provision or the recipients, among others, will require prior reporting.
The improvement of the security review is another significant provision in the Implementation Rules. While the HGR Regulations only stipulate that security review should be conducted when the provision of information might affect public health, national security, and public interest of China and provides corresponding penalties, the Implementation Rules elaborate the applicable scope and procedures of security review, as follows:
1. Applicable scope of security review:
Human genetic resource information of important genetic families;
Human genetic resource information in a specific area;
Human exome sequencing and genome sequencing information resources of more than 500 individuals;
Other situations that may affect public health, national security, and public interest of China.
2. Procedures of security review:
Formulate security review principles and establish an expert pool: MOST, in collaboration with relevant departments, will formulate security review principles, establish an expert pool, and enhance the expert administration systems;
Conduct security review: Randomly select review experts from the expert pool and conduct security reviews through online reviews (under normal circumstances) or through meetings, on-site inspections, and other methods;
Security review decision: MOST, in collaboration with relevant departments, will organize experts from relevant fields to conduct security reviews and make review decisions based on the security review opinions.
Administrative supervision and penalty
I. Enforcement requirements
The Implementation Rules reflect the ongoing trend in China of continuously emphasizing and strengthening the regulation of human genetic resources. Since the promulgation of the HGR Regulations, MOST has only published one administrative penalty case, which involved sanctions for submitting false application materials to obtain approval. However, from the provisions of the Implementation Rules, it is evident that the future regulation of human genetic resources in China will display a stronger regulatory intensity. It can be observed that from Article 57 to Article 61 of the Implementation Rules, detailed arrangements have been made for the supervisory and inspection work of the competent authorities, including the annual supervision and inspection plan, key supervision and inspection requirements, random supervision and inspection arrangements, specific supervision and inspection actions, and the supervision and inspection information archiving. This signifies a clear strengthening of the trend towards enhanced enforcement.
The Implementation Rules further refine the enforcement focus of the regulatory authorities regarding human genetic resources. Notably, the second paragraph of Article 56 in the Implementation Rules supplements the focus of supervision and inspection for human genetic resources regulation regarding "the exportation, external provision, open utilization and usage after exportation of materials or information". This addition, compared to the Draft Rules, highlights the regulatory authorities' focus on the external provision of human genetic resources. In the future, the industry should pay more attention on fulfilling compliance obligation when engaging in human genetic resource-related activities, with particular attention to the requirements of prior reporting before providing human genetic resource information to foreign parties.
While strengthening enforcement, the Implementation Rules also adhere to the regulatory authorities' targeted approach as demonstrated through past communications and practices. MOST clearly emphasizes in the document Policy Interpretation of the Rules for Implementation of Regulations on the Administration of Human Genetic Resources that, while firmly safeguarding national biosecurity, the administration of human genetic resources shall adhere to the principle of "stringent regulation where necessary and reasonable flexibility where appropriate". Additionally, Article 66 of the Implementation Rules emphasizes that the regulatory authorities should standardize the exercise of administrative penalty discretion, ensure penalty proportionality, and prevent over-punishment or under-punishment. Furthermore, MOST will formulate and publish separate discretion criteria for the administrative penalty of human genetic resources, which deserves further attention.
II. Penalty calculation basis
"Illegal income" serves as the basis for calculating fines under the HGR Regulations. However, the current HGR Regulations do not provide a specific definition of "illegal income". The Implementation Rules further specify the concept of "illegal income" by stipulating that it should be calculated by deducting reasonable expenses from the total income obtained through the implementation of illegal activities; and if such calculation is impractical, the value of the human genetic resources involved in the illegal activities or the amount of funds invested in those resources can be used as a basis for determining the amount of the fine. Compared to the Draft Rules, the Implementation Rules prioritize the calculation method for determining "illegal income" by deducting "reasonable expenses" from the "total income". Given that the income obtained from the utilization of human genetic resources is usually relatively limited, the fines are more likely to be reasonable in amount. This provision is consistent with the enforcement principle of preventing over-punishment or under-punishment, as stipulated in Article 66 of the Implementation Rules mentioned earlier.
Conclusion
The Implementation Rules have further refined and implemented the relevant regulatory provisions of the HGR Regulations, taking into account the industry's concerns and practical needs. From the provisions of the Implementation Rules, it is evident that regulatory authorities are dedicated to implementing a regulatory approach that firmly safeguards national biosecurity while effectively adhering to the principle of "stringent regulation where necessary and appropriate flexibility where applicable": On the one hand, the Implementation Rules optimize many administrative approval and filling requirements and procedures for human genetic resources-related activities, facilitating the fulfillment of compliance obligations for the industry and alleviates their compliance burden. On the other hand, the Implementation Rules strengthen the necessary regulatory force by specifying administrative requirements and implementing supervision and inspection measures, leading to a significant enhancement of the trend towards regulatory enforcement.
The finalization of the Implementation Rules signifies a new phase in the regulation of human genetic resources in China, and the regulatory requirements outlined in the Implementation Rules deserve due attention from the industry. We will also actively participate in discussions with regulatory authorities and industry entities, accompanying the industry in comprehending and acknowledging this regulation, as well as China's constantly evolving regulatory requirements for human genetic resources. With the continuous enhancement of regulatory measures concerning human genetic resources, we are committed to assisting the industry in effectively utilizing China's human genetic resources and facilitating the smooth operation of their business activities.
Important Announcement |
|
This Legal Commentary has been prepared for clients and professional associates of Han Kun Law Offices. Whilst every effort has been made to ensure accuracy, no responsibility can be accepted for errors and omissions, however caused. The information contained in this publication should not be relied on as legal advice and should not be regarded as a substitute for detailed advice in individual cases. If you have any questions regarding this publication, please contact: |
|
Aaron GU Tel: +86 21 6080 0505 Email: aaron.gu@hankunlaw.com |
[1] Shuwen Sun and Leyi Wang have contributions to this article.